Based on the clinical program strategy, we help you to define a supply chain strategy aligned with the objectives of your clinical program that will generate substantial benefit and cost saving.
Example of elements to be considered
These activities are paramount for the success of a clinical program and is most of the time part of the clinical supply chain activities. As the labelling rules and languages may differ by country, a coordination with other stakeholders (regulatory, CRO) should be done.
We also help you to identify and select the best secondary packaging partners.
Depending on the location of the DP production sites and the clinical dispensing sites, we help you to determine an effective distribution network composed of secondary packaging sites, warehouses and parcel distribution partners.
Thanks to our extensive network, we can help you to identify and select the best distribution partners according to your expectations and the clinical project specificities.
To ensure a full compliancy, a structured and flexible kit-list is paramount in any clinical trial.We have the expertise and experience to develop a kit-list that allow you the flexibility to generate additional sequence ranges if needed and with an adequate numbering structure.
Our experience shows us that the logistics and supply chain are not always fully considered in the configuration of the IWRS system. If we are included early enough in the configuration team , we make sure that the supply chain aspects are fully covered . It will ensure a smooth transfer of information along the value chain and keep you immediately informed of any movement or issue through adequate reports.
Of course, our project managers are experienced enough to drive clinical supply chain setup according to your needs and study specificities. Nevertheless, they are also field project managers that will follow the daily operations together with internal and external stakeholders. Clinical trials cannot afford any delay in decision-making processes.
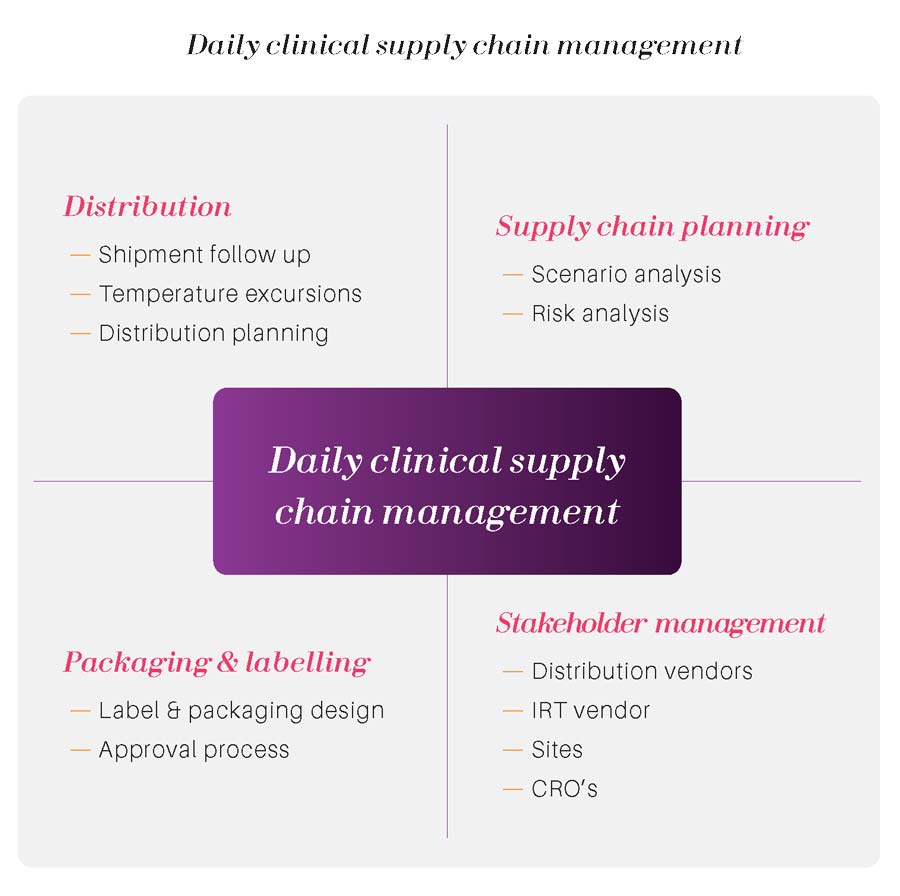
Of course, we are very happy to provide you long term support. However, in many case, our client needs our services to initiate supply chain processes and to structure their supply chain. In this case, the best scenario is to transmit our expertise to your own people to make sure that they will continue to generate substantial competitive advantage on the long run.
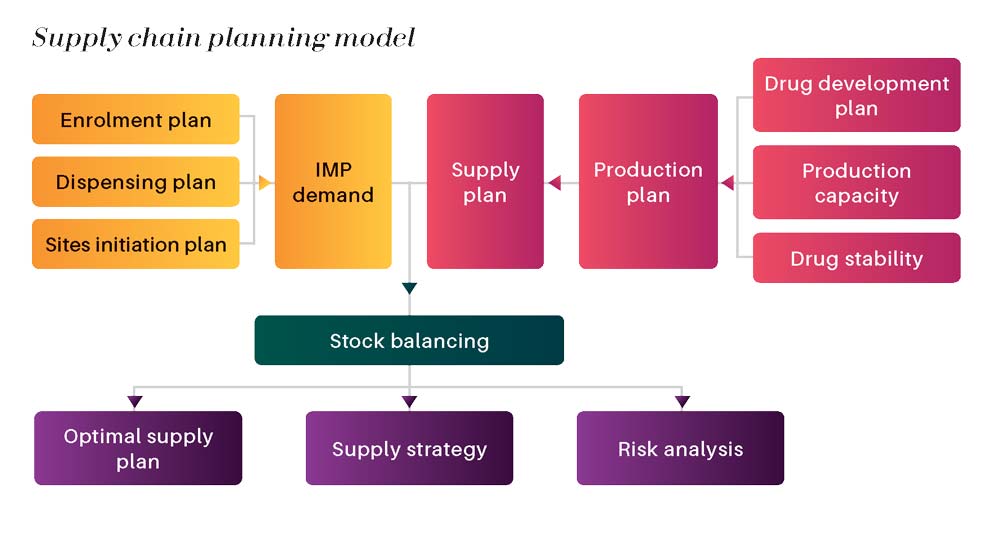
Recruiting and enrolling patients represents a huge budget of clinical trial programs. Being obliged to drop-off a patient due to an inventory shortage at site is an important hidden cost that can be avoided by an effective supply chain planning model.
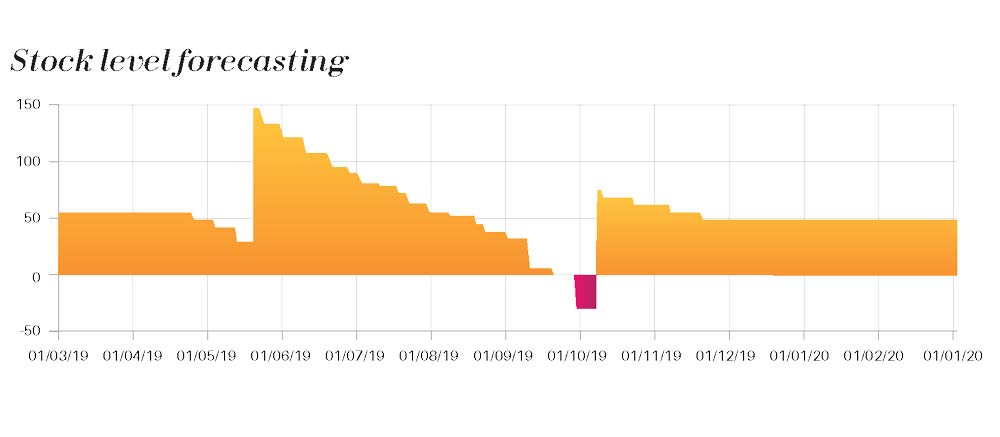
We incorporate all paramount variable data of the study (recruitment forecast, dispensing plan , production plan, site activation forecast, …) to build up a model that make sense and give a visual projection of the stock evolution according to various scenario that our client wishes to analyze.
The final deliverable of this D&OP exercise is in the form of a supply plan that supports the study program.
From the other hand and especially if the drug product is expensive, covering the risk by a stock buffer is not sufficient. Our role is also to propose a supply plan that avoid inventory shortage, but also optimizes production budget.
Beside the clinical supply chain services , Boostcode can also provide expertise and consultancy services in other areas of the clinical development such as regulatory management, quality management, CMC (Chemestry, Manufacturing and Control), external manufacturing, organization & HR, …
Don't hesitate to send us a message. Our team will contact you within 24 hours.